



Was ist “SaaS+”?
Die EU fordert, dass jede einzelne Medikamentenpackung fälschungssicher ist und zum Hersteller zurückverfolgt werden kann. Dazu wird jede Packung mit einer individuell verfolgbaren Seriennummer und einem Barcode versehen. Durch viele gesetzliche Vorschriften und investitionsintensiven Maschinen ist die Serialisierung für einen Hersteller sehr aufwendig und zusätzlich ist spezielles Know-How erforderlich. Zusammen mit seinen Partnern bietet MSK Serialisierung als flexible Dienstleistung an, die Sie fließend in Ihre Logistikkette integrieren können. Diese “Serialisierung as a Service” nennen wir “Saas+”.
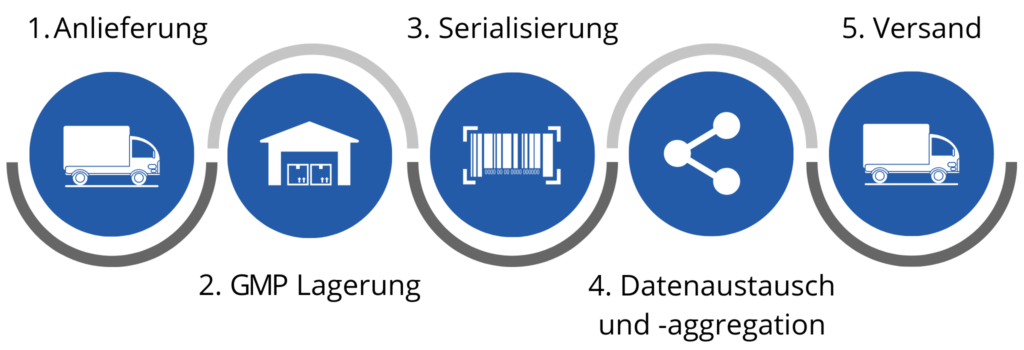
So funktioniert “Serialisierung as a Service”
Ihre Produkte werden in unser GDP- und GMP-zertifiziertes Lager am Standort Heppenheim angeliefert. Dieses liegt verkehrsgünstig in der Metropolregion Rhein-Neckar. Dort werden die Verpackungen serialisiert. Unsere Maschinen sind so flexibel, dass wir alle Packungsgrößen und Stückzahlen bedienen können. Die Daten werden über sichere Systeme und gemäß der europäischen Datenschutzvorschriften in das European Medicines Verification System eingespeist, sodass jederzeit überprüfbar ist, ob dieses Medikament echt ist und woher es stammt. Danach werden die Produkte wieder in Ihre Lieferkette eingegliedert und weiterverteilt.
So werden die Vorschriften der EU-Pharmarichtlinie ohne großen Aufwand erfüllt. Außerdem können Sie unterschiedliche Mengen nach wechselndem Forecast bedienen und bezahlen ausschließlich pro Stück und ohne Fixkosten.

Welche Vorteile bietet SaaS+?

100% SKALIERBAR
Wir bedienen alle Stückzahlen, da wir durch unsere Partner exklusiven Zugang zu Maschinen haben. Und wenn sich Ihr Forecast nächsten Monat ändert, passen wir uns mit an.

KOSTENGÜNSTIG
Statt die investitionsintensiven Maschinen zur Serialisierung selbst zu beschaffen und eigenes Personal einzustellen und auszubilden, profitieren Sie von unseren Skaleneffekten.

VARIABLE KOSTEN
Wir berechnen Ihnen keinerlei fixe Gebühren. Alle Kosten fallen pro serialisierter Packung an, sodass Sie ausschließlich variable Kosten haben.

GMP ZERTIFIZIERT
Als Experte in der Pharmalogistik sind alle Lager und Prozesse bei MSK Pharmalogistik natürlich GDP und GMP zertifiziert.
Lassen Sie sich jetzt unverbindlich beraten und finden Sie heraus, wie SaaS+ Ihrem Unternehmen helfen kann.
Was sagen unsere Kunden über “Serialisierung as a Service”?
Wir besitzen für die Lagerung und den Versand von Healthcare-Produkten eine umfangreiche Logistikinfrastruktur mit 4.500 m² Lagerfläche, die speziell und ausschließlich für die strikten Anforderungen der pharmazeutischen Industrie gebaut wurde.
Wir besitzen für die Lagerung und den Versand von Healthcare-Produkten eine umfangreiche Logistikinfrastruktur mit 4.500 m² Lagerfläche, die speziell und ausschließlich für die strikten Anforderungen der pharmazeutischen Industrie gebaut wurde.
Wir besitzen für die Lagerung und den Versand von Healthcare-Produkten eine umfangreiche Logistikinfrastruktur mit 4.500 m² Lagerfläche, die speziell und ausschließlich für die strikten Anforderungen der pharmazeutischen Industrie gebaut wurde.
Wir besitzen für die Lagerung und den Versand von Healthcare-Produkten eine umfangreiche Logistikinfrastruktur mit 4.500 m² Lagerfläche, die speziell und ausschließlich für die strikten Anforderungen der pharmazeutischen Industrie gebaut wurde.
Das Team von SaaS+

Die MSK Pharmalogistic GmbH ist der Full-Service-Dienstleister der Pharmabranche für Logistik, Lohnverpackung und Dienstleistungen in den Bereichen Personal, Vertrieb und Marketing.

Die Metacarp GmbH bietet innovative und ganzheitliche ERP-Lösungen nach GMP. für die pharmazeutische Industrie an. Dazu zählt z.B. Produktion, Qualitätssicherung, Chargenrückverfol-gung und sofort einsetzbare Software für die Serialisierung.

Die tracekey solutions GmbH ist Experte für kompakte und flexible Serialisierungsprojekte in der produzierenden Industrie. Ihre IT Lösungen fokussieren sich auf höchste Transparenz für den ganzen Product Life Cycle.

Die Laetus GmbH ist mit ihren modularen Verpackungs- und Supply-Chain-Lösungen Branchenführer für inline Qualitätskontrolle und hauptverantwortlich für die Entwicklung von Secure Track & Trace Solutions (S‑TTS).
Häufig gestellte Fragen zu SaaS+
Sie haben die volle Kontrolle. Wir laden uns die gewünschten Seriennummern aus Ihrem Level‑4 System herunter und übermitteln Sie im Anschluss der Produktion wieder.
Wir verwenden verschiedene Methoden um die Verpackung zu verschließen. Ob mit In-line Etikettierer für Tamper Evident Sticker oder durch manuelles bekleben mit Heißkleber. Ebenfalls verwenden wir, wenn gewünscht, Ihre beigestellten Etiketten.
Bevor der erste Auftrag von uns gefertigt werden kann, muss im Vorfeld der Prozess abgeglichen und Sie in unser Systemlandschaft integriert werden. Dies ist einmalig notwendig und ist produktunabhängig.
In der Regel können Maschinen nur gewisse Größen bedrucken. Durch den Einsatz eines eigenen Serialisierungs-
Wir können die Serialisierung gemäß EU-Richtlinie, aber auch Russia oder UDI für Sie vornehmen. Andere Serialisierungsformen können natürlich nach Anforderungsübermittlung mitaufgenommen werden.

Lassen Sie sich jetzt unverbindlich beraten und finden Sie heraus, wie SaaS+ Ihrem Unternehmen helfen kann.
Impressum Datenschutzerklärung
© 2019, MSK Pharmalogistic GmbH. All rights reserved.

